What is an Atom?
An atom is a remarkable example of how stable oscillating instances can form resonances with other stable instances.
Resonances arise between rotating electrons and their counterparts. The more resonances they find, the stronger their mutual bond becomes, and the closer the electrons arrange themselves around each other.
Resonant Structure
The atomic structure will show that the planar, rotating singular entities we have considered so far now adapt into new configurations.
In these configurations, additional resonances appear that exist only within a combined network of resonant entities.
For now, let us fill up the electron orbitals of an atom while ignoring the nucleus and treating it merely as the central anchor for each electron’s bonding.
Stepwise Filling of Orbitals
-
1–2 electrons
Simple — let both rotate in a single plane around the center.
-
3–4 electrons
Now we have two Rotons (each consisting of 2 electrons). Let them rotate co-axially, which produces the strongest attraction.
Assign their shells (orbitals) suitable distances so that their oscillations can resonate — their angular velocities being harmonic multiples.
For e.g. the beryllium atom with 4 electrons, this corresponds to radius 1 for the inner shell and radius 4 for the outer one.
-
5–10 electrons
When we add two more electrons, they will seek positions that maximize the number of possible resonances.
They can couple to both existing shells, so the new electrons will oscillate with two resonance components — one corresponding to $\omega_1$ (inner resonance) and one to $\omega_2$ (outer resonance).
Although they likely occupy the second shell (as electrons 3 and 4 do), their motion may combine a radius of shell 2 in the x-direction with a radius of shell 1 in the y-direction.
More precisely, an electron can rotate in the second shell while simultaneously precessing with the magnitude characteristic of the inner shell.
This hybrid resonance pattern can be visualized in several equivalent ways, which we will explore in the following chapters.
Goal
The goal is to demonstrate how a simple and intuitive model can explain the fundamental structure and properties of atoms.
Visualization of resonance coupling via precession:

Resonance Relations
The harmonic coupling between the first two shells (1s, 2s) can be expressed as:
$\frac{r_2}{r_1} = 4 \qquad \text{and} \qquad \omega_2 = \frac{\omega_1}{4}$
These ratios define the stable resonance condition for the first two shells and set the basis for higher-order orbital configurations.
*The smallest harmonic ratio $\\omega_2 / \\omega_1 = 1/4$ is chosen in the examples, because it represents the simplest **phase-locked resonance**, where four inner oscillations complete exactly one outer rotation. In contrast, the quantum-mechanical Bohr model predicts $\\omega_2 / \\omega_1 = 1/8$, though the radius ratio $r_2 / r_1 = 4$ remains consistent in both frameworks.*
This slower 1/8 relation allows the outer electron to sweep through all resonance phases of the inner oscillation. It allows even a single electron to dynamically reach all spatial extensions of the 2p orbital — consistent with experimental observations of orbital field coverage and electron density distributions. The resonance coupling model (RCM) will take up the 1/8 relation later on.
Rotonal Olavian Atom Model
We introduce the “Resonance Coupling Atom Model (RCM)” or in short “Olavian Atom Model” describing the existence and properties of atoms within the periodic system of atoms.
This is a proposal, how atoms and their structure and bonding might be explained on basis of the Quantum-Roton-Model in a much more intuitive way.
Optimization
The universe is a constant wobbling of stable resonances approaching an energetically optimal placing in space. More levels of rations and resonances result in more attraction and a better energy distribution. So optimal states are reached by random background noise or external energy/influences disturbing the current minima.
A simple atom model can initially only describe a part of what is an optimal placement of Electrons, Protons and Neutrons - until other more optimal resonances are found. This even holds for the same atom which might favor different structures in different situations and environments (single atom, excitation, molecules).
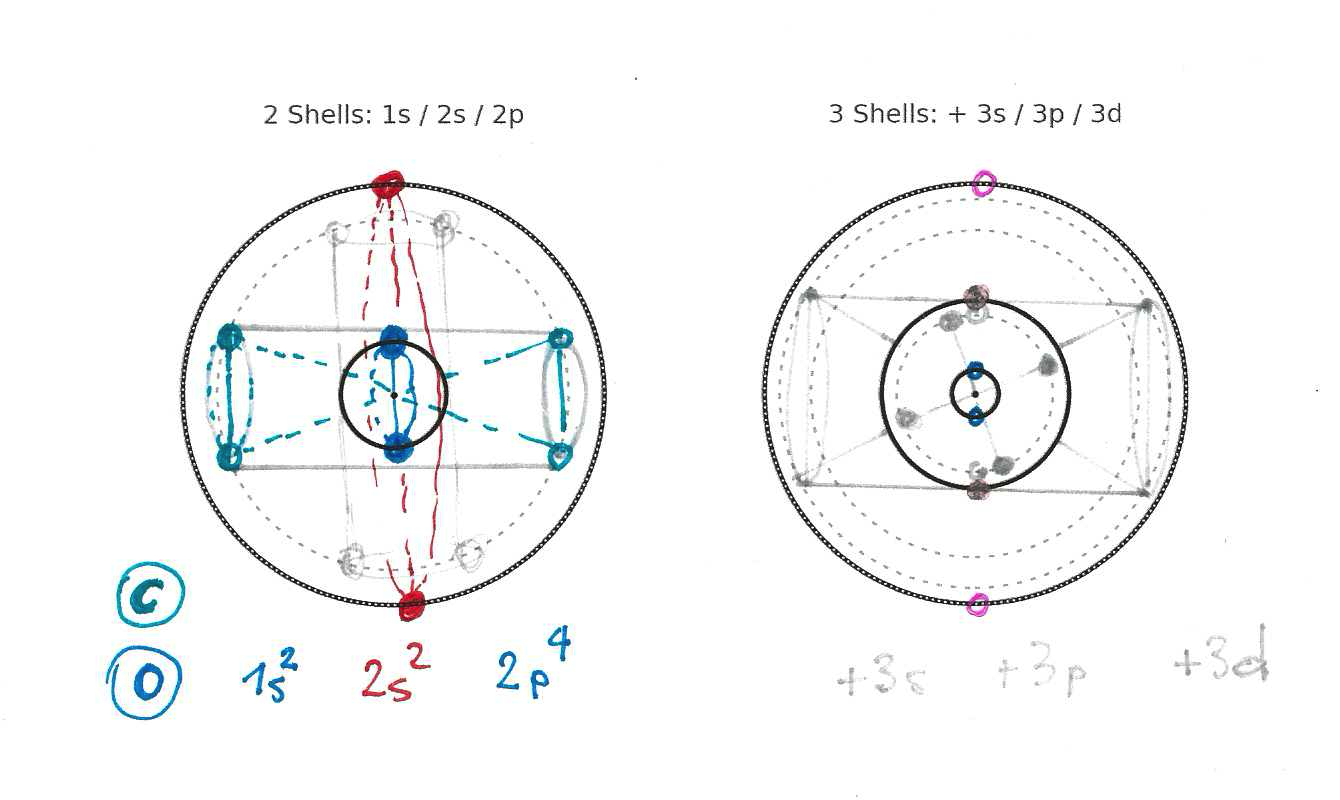
Atom structure TRY 1
Let’s start with a FIRST TRY (not ground state) to give you a glimpse on how a more optimal placing solution can be found. These are some original notes on this topic - FIRST TRY (not accurate):
Initial try - attention: this is not the optimal ground state of an atom:

This illustration shows protons and electrons (neutrons are omitted for now) and how they build electron-pairs via entanglement (long distance co-axial rotation). The valence-electrons (sketched in magenta) do not have a sparing electron partner and therefore can not reach a nearer location to the center. The attraction is never-the-less created to the proton. So there are pairs of entangled electron-proton pairs and electron-proton-proton-electron double-pairs.
Important note: After first discarding these atom structures very soon, I realized, that many or similar variants of the shown atom structures actually do exist - but they are not the energetically optimal solutions (ground state) so they will decay into more stable configurations. But as they do exist, I allow myself to show them at this place, as intermediate excited versions of the base atom states. Intermediate states in the transition to finding the most energetically optimal configuration (best energy density, closest to the nucleus).
This can be visualized more closely in the following figures showing three atoms.
- Helium atom He with order number 2 (2 electrons, 2 protons)
- Berilium atom Be with order number 4 (4 electrons, 4 protons)
- Carbon atom C with order number 6 (6 electrons, 6 protons)
OUT OF DATE !!! this is from an older investigation state which did not respect resonant orbitals
| Helium (2) |
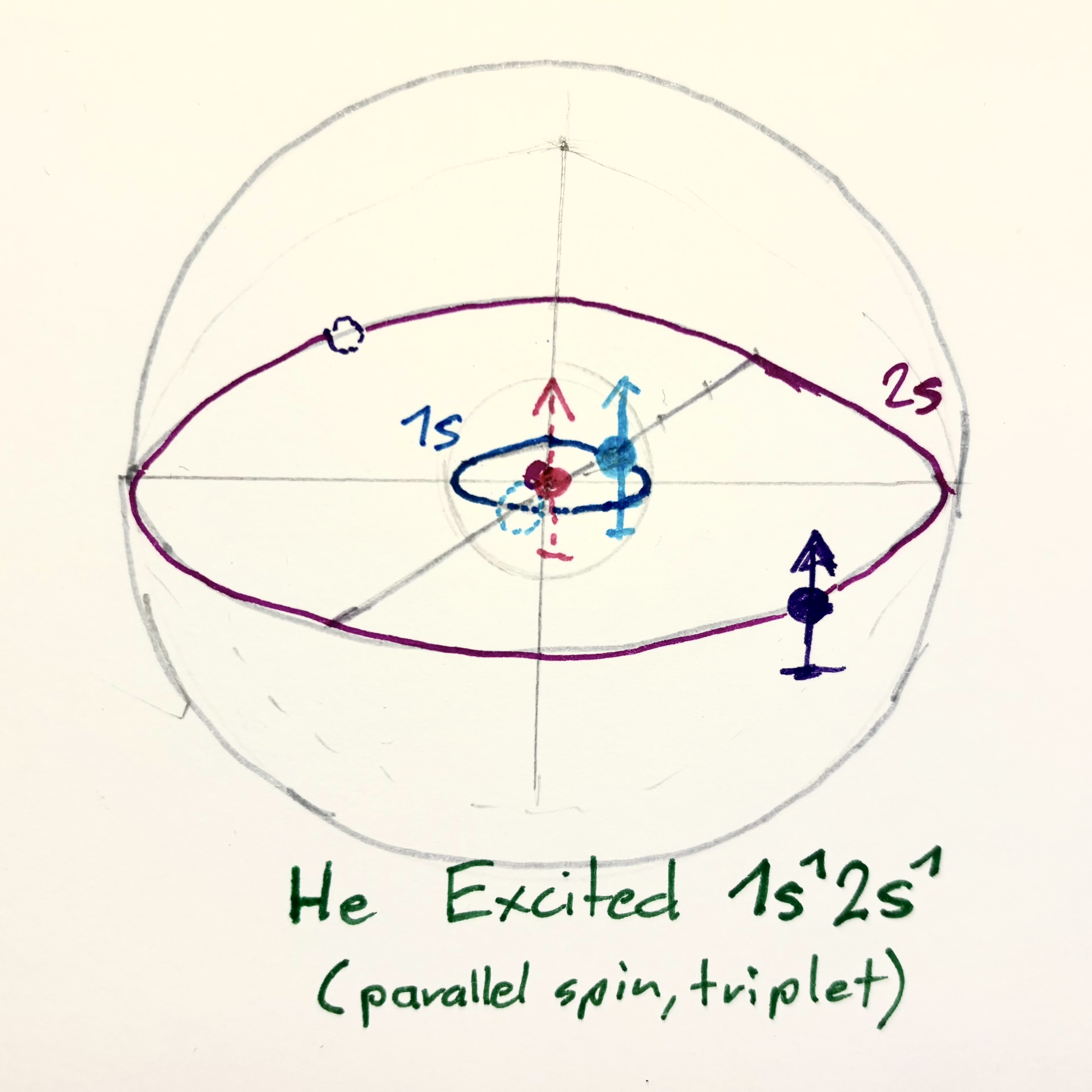
Berilium (4) - UNSTABLE Excited $\text{Be}^* : 1s^0 ; 2s^4$ ($1s^0 ; 2s^2 2p^2$) |
($\text{Be} : 1s^2 ; 2s^2$) ??? |
Carbon (6) - UNSTABLE Excited $\text{C}^* : 1s^0 , 2s^2 , 2p^4$ |
 |
 $- - >$ $- - >$ |
 |
 |
These figures basically show a proposal, where electron-pairs rotate in separate parallel planes (co-planar). This leads to attractive forces between these rotations which binds the electron-pairs closer to each other. BUT the planes will eventually align into one plane caused by their attractions. So this might not be a stable final solution.
The electron spins are co-axial pointing into the center of the atom. This seems to result in the most optimal attraction. We are not going into discussion of nucleus structure here, but further calculations show, that the shown Carbon Roton-Structure does not result in the energetically most optimal configuration. Therefore the shown model of the Carbon-Atom is discarded as the ground state atom. We might most likely need go to structures with rotations of different radii.
The atom models shown above do actually exist in the Bohr-Atom model as excited states:
What happens in physically in excited atoms (Bohr Model)
Neutral ground-state beryllium has configuration: $\text{Be: } 1s^2 ; 2s^2$
If both 1s electrons are excited into the 2s shell, the configuration becomes: $\text{Be}^* : 1s^0 ; 2s^4$
That means all four electrons occupy the same principal shell (n = 2) — an energetically unstable state that quickly decays back to $1s^2 2s^2$.
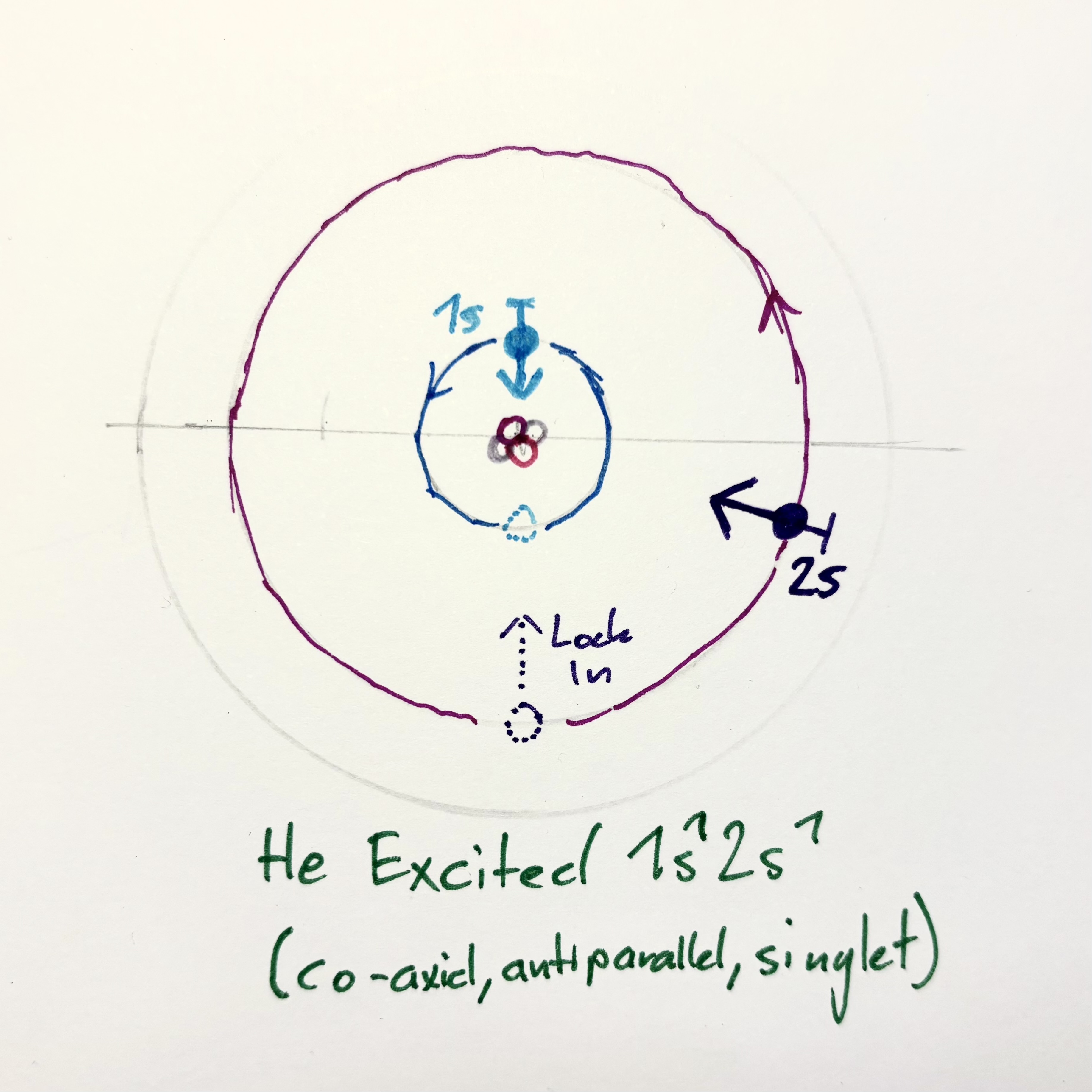
Ground-state carbon: $\text{C: } 1s^2 , 2s^2 , 2p^2$
Excited configuration (promote both 1s → 2s or 2p): $\text{C}^* : 1s^0 , 2s^2 , 2p^4$ (with sub-shells actually $1s^0 ; 2s^2 2p^2$)
In an excited carbon atom, both core electrons transition into the second resonance shell. All six Rotons now oscillate at the same harmonic domain ($\omega_2$), forming three mutually orthogonal orbital planes corresponding to the 2s and 2p families. The result is a spherical resonance lattice of six interacting oscillators — maximally coherent but energetically unstable, as the inner coupling $\omega_1$ vanishes.
When the system decays, two Rotons collapse back into the $\omega_1$ domain, re-establishing the inner stabilizing core of the atom.
What is missing in our model?
(A) Radial resonances (varying radii)
This model misses the fact, that rotations of different sizes still might have resonances. If the frequencies of two rotations have a common integer divider, they might create resonant attractions. The distances and speeds of the rotating electrons need to match these condition in respect to their attractive forces.
These resonances might apply, when the LEDO-waves build full multiples in respect to their frequency and rotation-phases within a plane. If we calculate the distances between these rotation-shells, we quite easily end up with the standard physics boor-atom model shell structure. You can find the calculations for the resonant placements further down. Here is the resulting formula: $r_n = \frac{r_1}{n^2} .$ The shell radii correlate to the inverse square of the resonance index.
How nice: From our own considerations, we have derived the shell distances of the standard boor atom model in physics.
 |
 |
 |
 |
| Resonance Distance of 2 shells |
3 resonant shells (+ sub-shells) |
Integer multiple phases |
Multiples in circumferences |
This illustrations show:
- 1: The shell distances calculated with rotation resonances where the frequencies share common integer multiples.
- 2: More main-shells. Some sub-shells. Some calculations for further partially harmonic Sub-Shells are also shown. This concept is not used further on though.
- 3: A symbolic visualization of integer multiples in rotations.
- 4: Symbolic resonances shown with N-point figures.
With this in mind, we have to place the electrons on orbitals which show this additional resonances in case of “co-axial” rotations.
Shell Hypothesis: While entering the world of multi shell electron orbits, the orbits will share an integer multiple of their frequencies.
(B) Bi-Resonant coupling
After having entered the world of different rotation radii (3-4 electrons) any further atoms might want to synchronize with any of the present electron rotations to reach an energetically more optimal state.
Bi-Resonance Hypothesis: Electron rotation-paths will try to have a resonant frequency with as much other electrons as possible (mostly with 2 other shell main-radii). We now open the atom model into the 3 different spatial directions.
Basically we expect, that a new shell will first contain a 2-electron Roton main shell (1s, 2s, …) before the harmonic sub-shells are filled (2p, 3p, …). Otherwise there would be no resonance.
Atom structure TRY 2
The following illustration shows a more optimized and more detailed possibly energetically better possibility for rotational electron positions. In addition we take up the idea of multiple parallel planes with similar rotation radii but bi-resonant coupling (to multiple $_omage$). These plains have a main rotation (the biggest) and some precession in the other spatial directions. This makes sense as parallel rotating planes attract each other so the electrons are attracted towards the second plane resulting in some oscillation around the main plane.

This model only shows the first 2 main resonance shells, potential sub-orbitals are not shown (energy level).
Examples:
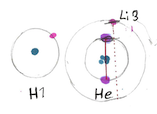
- H: A single Electron rotating around the central nucleus (nucleus is not shown).
- Li 3: Two electrons in the first inner shell and one single electron on the second outer shell. Up to 4 electrons mmight share the same rotation plane, keeping the co-axial entanglement with the core.
- C 6: This atom with 6 electrons starts to get interesting. Now the inner two electrons build a further perfect rotation partner for the further outer electrons in X-Axis.
- O 8: Now this gives a very nice view onto a similar additional electron-pair with rotations in Y-Axis (*extension)
- (Ne 10): Not shown (adding the Z-Axis)
( *extension): experiments show that x,y,z are first filled with 1 atom. This might therefore result in better resonant attractions
We are currently looking at 3 Levels of electron placement:
- 2 at innermost shell (1s), in blue
- 2 in the second shell (2s), in red
- 2, 4 or 6 in the third shell (2p), in green most likely slightly closer to the center than 2s, caused by more rotation resonances.
With this in mind we can think of the following extensions (spoiler):
| Electrons |
Atom |
shell (s) |
bi-resonant sub-shells (p) |
Resonances |
Added e- |
Total filled Atom electrons |
Roton plains (*) |
Comment |
| 2 |
He |
1 (1s) |
|
1-0-0 |
|
s*2 = 2 |
:1| |
|
| 4 |
Be |
2 (2s) |
|
2-0-0 |
+ 2 |
s*2 = 4 |
:1|1: |
|
| 10 |
Ne |
2 |
1 (+2p) |
2-1-0 |
+ 6 (2 per axis) |
s*2+p*6 = 10 |
:1|:2:3:2: |
p2 orbitals, sync s2 and s1 |
| 12 |
Mg |
3 (3s) |
|
3-0-0 |
+ 2 |
s*2+p*6 = 12 |
:1|:2:3:2:|1: |
|
| 18 |
Ar |
3 |
2 (+3p) |
3-2-0 |
+ 6 |
s*2+p*6 = 18 |
:1|:2:3:2:|:2:3:2: |
p3 orbitals, sync s3 and s2 |
| 20 |
Ca |
4 (4s) |
|
4-0-0 |
+ 2 |
s*2+p*6 = 20 |
:1|:2:3:2:|:2:3:2:|1: |
|
| … |
… |
|
|
|
|
|
|
|
| order ? |
… |
4 |
2p + 3d (xy, xz, yz) |
3-1-0 |
+ 6 (2 per axis) |
Ca + 6 ? |
Ar+2/2/2/ |1: |
2D sync of s3 with s1 |
| order ? |
… |
4 |
2p + 3d (xyz) |
3-2-1 |
+ 2 |
Ca + 6 + 2 ?? |
+|.010.|… |
3D sync of s3 with s2 and s1 |
| order ? |
… |
4 |
2p + 3d (xyy) |
3-2-2 |
+ 2 |
Ca + 6 + 2 + 2 ?? |
+|.101.|… |
3D sync of s3 with s2/s2 |
| 36 |
Kr |
4 |
2p + 3d + 3p |
4-3-0 |
+ 6 (3p) |
Ca + 6 + 2 + 2 + 6 |
:1|:2:3:2:+:111:|:2:3:2: +2/2/2/|:2:3:2:|1: |
2D sync of s4 with s3 |
| … |
… |
… |
… |
… |
… |
… |
|
further combinatorics |
(\*) *Resonance notation:* x-y-z where x,y,z are the shell numbers to which there is a resonance in the corresponding spatial direction
(\*) *Roton Plain notation:* This n|m|o|p indicate the number of plains in the corresponding shell 1-4 (1|2|3|4). The notation ":1" is a single shell with 2 electrons. The notation ":x:z:y:" indicates the number of Rotons/planes in the corresponding spatial direction with a "." for each electron. So ":2:3:2:" is a shell with 1 middle Roton (2s) and 2 additional planes per spatial direction with one electron each and a total of 8 electrons.
Sub-Shell Rotation Type
What makes the rotations in the green shells special? They are rotations around at least 2 axis in space. Why is that?
The issue with an electron rotation in a parallel plane to the s-planes is, that the electron has no entanglement to any proton if two electrons would rotate at that place. So the electron still has to couple to a proton and a potential other paired electron would need to be placed on the opposite side of the nucleus. So in the end, the electron in a 2p shell first rotates along the X-Axis as the two 1s electrons. And second it also rotates around the nucleus giving the overall rotation a rotating 2-cone structure.
Going into 3D. Now this same rotation could happen in the direction of the Y-Axis which is shown in the O-8 (oxygen) atom. The electrons can rotate without getting each other into their way. You might surely give a solution for filling two further 2p electrons into the Z-Axis cones.
Having a look at the standard physics atom model shows, that the shells and sub-shells we just introduced within the roton-model have standard names (1s, 2s, 2p). Even the number of electrons per shell give a perfect match: 2 electrons in single plane, 2 co-axial electrons in second resonance shell, $3*2$ electrons per spatial 3D direction.
When building molecules, the position of the 2p electron “Rotons” might partially be a little closer to the perimeter of the atom. So they will provide the most relevant valence electrons and will most likely do the bindings also with an odd number of electrons in the p-shells. So also in molecules the s-shells are most likely filled with the 2 electrons before the p shells. The s-shells are needed fully filled for providing the resonance layer. Or in other words the 1s/2s rotons will realign to resonate optimally with the 2p electrons. So 2p electrons might be slightly farther away from the center than the 2s electrons.
The Roton model would intuitively predicts though, that for single isolated atoms, a single (unpaired) valence electron (e.g. for the N-Atom or F-Atom) might rather be placed on the 2s shell. But this would mean, that enough 2p self-resonances would need to be present with no need for a full 2s resonance.

Will the s or p shell be first filled up in an atom? It will be the one, which gives the system more energy - and most likely draws parts closer to the center.
It is difficult to tell what “closer” means:
- Most probable distance: 2p is closer to nucleus.
- Average distance: 2s slithly smaller
- Energy (binding strength): 2s (stronger nuclear penetration)
Result: This means, that the 2s shell is energetically better compared to the 2p shell and allows a “smaller” or rather more optimal atom structure. Even for isolated atoms. So the s-shell is always preferred to be filled up first.

Predictions:
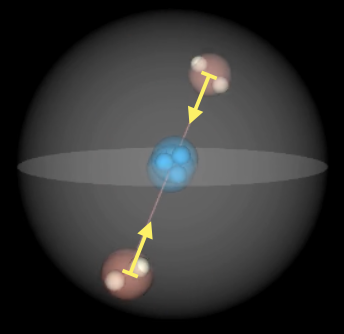
Elliptic: The atoms H to Be have the electrons placed more in a planar way than the other atoms (with p-shells). Verification: This is the case, if e.g. a He atom can be placed (polarized) within a magnetic field, which is only possible if an electron is excited. The resulting spin-polarized helium has a precession (Larmor frequency). This confirms the slightly elliptic shape if it can be polarized.
Further atom shells
Now what’s the next extension? We now have an Ne Atom with full shells: $1s^2 2s^2 2p^4$
Ok, let’s add the next 2 rotating electrons one resonance layer further. As we add only 1 or 2 electrons they fit into a further plane quiet well. No need for cone-like wobbling shells.
Next we can add the 3p (inner) double-cone electrons in their X, Y and Z rotation directions.
I’m honestly really exited about this match. Perfectly intuitively explaining why electrons might arrange themselves this way. NOT because of their repulsive character (which they do not have anyway, at least not more than between other electrons and protons) - but because the rotations create a higher level of attraction. This results in a more compact and energetically optimal placing and rotating of the electrons. All experiments so far indicate that the N-Atom has 3 unpaired electrons in the p-shell.

Orientation variants of Electrons in an atom
The Roton model can give consistent explanations which stable positions and orientation electrons take within an atom.
The most favorouble constellation is an opposite coaxial orientation of the electron rotation axis (spins) in which they reach an energetically optimal constellation.
Please find the full detailed descriptions here
Layering of electron orbitals (Ledoigtian atom shell model)
Basic considerations:
- An optimal stable structure is reached, when electron rotations have a phase which aligns over time to a base phase.
- Every electron-pair is identically attracted to the center with the same distance independent force. This is supported by an “Electron <-> 2 Proton <-> Electron” entanglement.
- Every odd valence electron is attracted to the nucleus with a constant distance-independent force (Electron <-> Proton entanglement).
Resulting orbital structure:
Oscillation Version:
We define: $x(t) = f_x(t), \quad y(t) = f_y(t), \quad z(t) = f_z(t)$
where each f_i is an independent 1D oscillation (e.g. sinusoidal, resonant, with its own frequency ωᵢ and phase φᵢ).
This means the total “state” is a product of three orthogonal oscillators:
$\mathbf{r}(t) = (f_x(t), f_y(t), f_z(t))$
In total this gives the following path for a single point in space:
$r(t) = A_x \cos(\omega_x t + \phi_x) + y(t) = A_y \cos(\omega_y t + \phi_y) + z(t) = A_z \cos(\omega_z t + \phi_z)$
With amplitude $A_n$, relative frequency $\omega_n$ and relative phase $\phi_n$ in each direction $n={x,y,z}$
To make it a closed resonant figure we need to align $\omega$ such that the frequencies are integer multiples.
To make them continuous figures we also have to adjust the relative phases in some matching way.
This generates Lissajous figures that form 3D standing or rotating loops — and those are analogues of complex-valued eigenstates in quantum mechanics. Three independent oscillations (x,y,z) with adjustable frequency ratios and phase offsets are the direct geometric analogue of Schrödinger’s separable wavefunctions. Playing with phases and ratios gives you angular momentum, quantization, and orbital symmetry.
🧩 Oscillator parameters for orbital analogues
| Orbital |
Resonant meaning |
Aₓ : Aᵧ : A_z |
ωₓ : ωᵧ : ω_z |
(φₓ , φᵧ , φ_z) |
Geometric / physical interpretation |
| s₁ |
fundamental radial mode (1s) |
1 : 1 : 0 |
1 : 1 : 0 |
(0 , 0 , 0) |
All axes in phase → single circular loop; isotropic “breathing” |
| s₂ |
2s with one radial node |
4 : 4 : 4 |
2 : 2 : 2 |
(0 , 0 , π) |
Same frequencies but opposite global phase → inner + outer shells (node between) |
| p₂ |
2p (one nodal plane) |
4 : 4 : 0 |
1 : 1 : – |
(0 , π , –) |
One axis 180° out of phase with others → two opposite lobes separated by a plane |
| s₃ |
3s (two radial nodes) |
9 : 9 : 9 |
3 : 3 : 3 |
(0 , 0 , π) |
Triple-frequency breathing → three concentric shells |
| p₃ |
3p (one major + one minor lobe) |
9 : 9 : 0 |
3 : 3 : – |
(0 , π⁄3 , –) |
Higher frequency, partial phase offset → three-lobe p-like shape |
| d₃ |
3d (quadrupole pattern) |
9 : 9 : 9 |
2 : 1 : 3 |
(0 , π⁄2 , π) |
Unequal integer ratios produce four-lobe or cloverleaf geometry with nodal planes |
Precession Version:
Rotonal Version:
Shell Radii Scaling in the Olavian Resonance Atom Model
Introduction
In the simplified Olavian Resonance Atom Model, electrons are particles on circular shells (at $r_n$) around a central nucleus.
Two guiding principles shape the shell structure:
- Constant central force: each electron experiences the same attractive force $F_0$ toward the nucleus, independent of its distance.
- Resonant phase synchrony: all orbital angular frequencies are integer multiples of a common base frequency $\Omega$, giving the atom a periodic stability and stable oszillations/resonances.
With these assumptions, the structure of the electron shells can be derived in a simple and elegant form.
Calculation
Please find the full detailed calculations here
Calculation Summary
Define the outermost shell (slowest rotation) with index $n=1$: $r_1 = \frac{F_0}{m \Omega^2}$
Then every other shell radius is given by the simple quadratic law: $r_n = \frac{r_1}{n^2} .$
This is the core result: the shell radii shrink inversely with the square of the resonance index $n$.
This exactly correlates with Boors standart model of electron shells.
Step 4: Derived orbital properties
Velocity relation: $v_n = \sqrt{\frac{F_0 r_n}{m}} = \frac{v_1}{n}$
Orbital periods: $T_n = \frac{2\pi}{\omega_n} = \frac{T_1}{n}$
The kinetic energy on shell $n$ is: $K_n = \tfrac12 m v_n^2 = \frac{K_1}{n^2}$
Because all frequencies are multiples of $\Omega$, the global atomic state is exactly periodic with common recurrence time: $T_{\text{global}} = \frac{2\pi}{\Omega} = T_1$
Discussion
- The model produces a $1/n^2$ law for shell radii, a simple resonance structure directly tied to the assumptions of constant central force and integer phase locking.
- Inner shells rotate faster and carry higher angular frequency, while their kinetic energies diminish as $1/n^2$.
- The entire atomic configuration re-aligns perfectly after the fundamental period $T_1$, ensuring stability of the resonant structure.
Conclusion
The Olavian Resonance Atom Model shows that even with a distance-independent central force, the imposition of integer phase synchrony is sufficient to generate a discrete shell structure. The natural outcome is a hierarchy of radii:
$$
r_n \propto \frac{1}{n^2} ,
$$
demonstrating how resonance principles can give rise to stable, quantized atomic configurations.
Size of atoms
Why do atoms have the size they have today? Because all others have, so they are in oscillatory resonance with each other which gives them the stability.
More precisely though, atoms receive their size based on the background fluctuations in the LEDO-field. Or on more standard terms by the cosmic microwave background (CMB).
Resonant origin of atomic scales
In this view, the cosmic microwave background (CMB) is not just a relic glow, but a continuous resonant field that defines the stable dimensions of matter.
When the primordial plasma cooled to about 3000 K, electrons and protons began forming the first bound atoms.
That temperature marks the threshold where the average photon energy dropped below the ionization energy of hydrogen — the moment when stable atomic structures became possible.
Since then, the universe has remained immersed in this CMB field.
Its radiation acts as a universal background resonance, fixing the characteristic energy scales at which electrons orbit, atoms remain stable, and electronic excitations begin.
In that sense, the sizes and energies of atoms today still reflect the equilibrium conditions established
at the epoch of photon–matter decoupling.
One more thought before we continue: The same could be told for the point in time, when the subsequent hydrogen and helium plasma expanded and started to form the first suns. Until then, no photon the size of a sun could emerge or pass through the universe. Only after the first suns formed and there started to exist “empty” space between the stars, then photons of that size could emerge. So we expect some CMB for that occasion in time too.
Distribution of electrons to shells
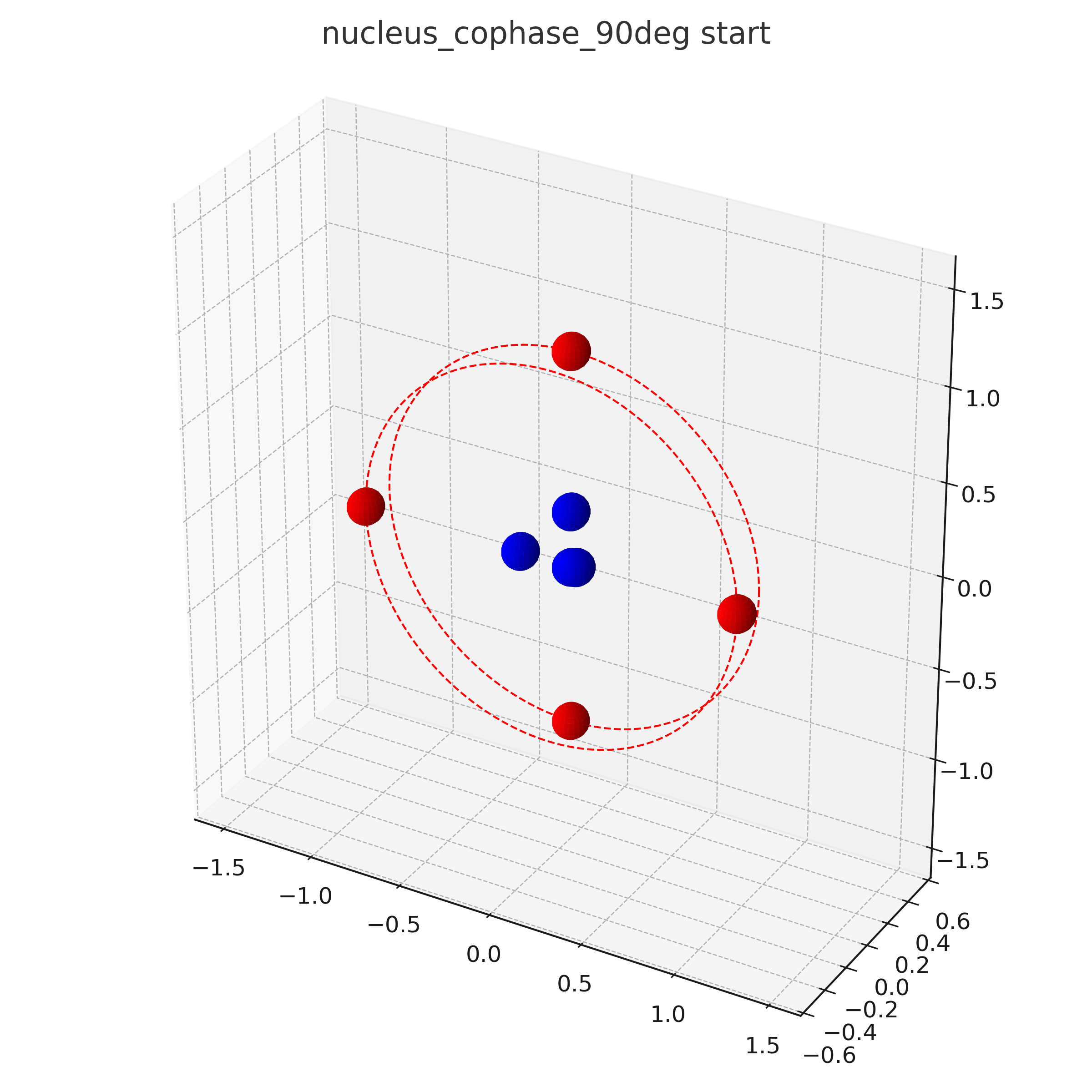
Animations
OUT OF DATE !!! this is from an older investigation state which did not respect resonant orbitals
For a more fancy view this is an animation of a Berilium atom Be:
And an animation of a Carbon atom C:
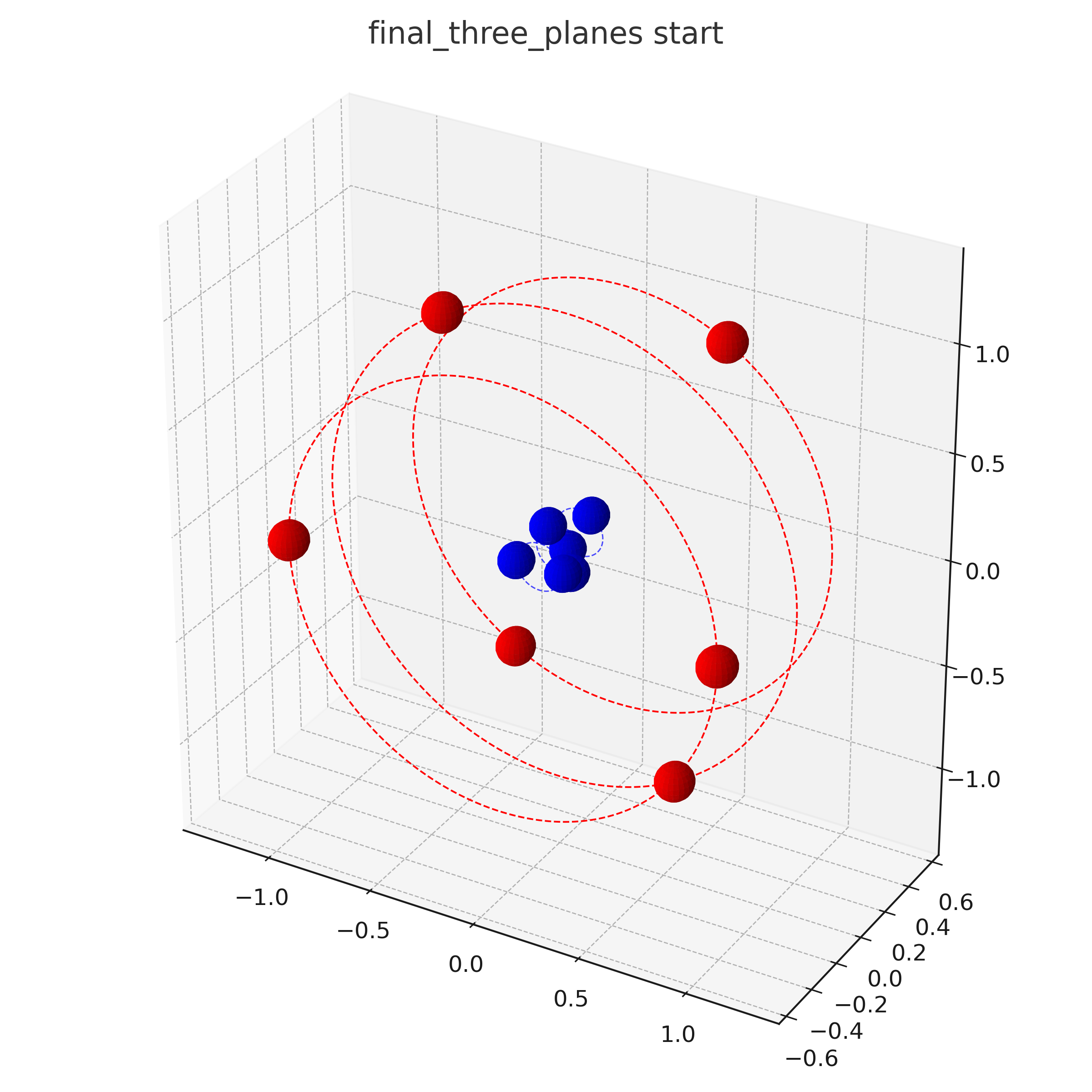
The following is not taken into account in these visualizations:
- Detailed Structure of the nucleus e.g. neutrons
- Rotation speeds of the electrons in the different planes. The center-plane in the C-Atom might e.g. rotate slower than the other planes.
Model rules
The rotonal model intends to show how the universe might be built up with the exact same rules on every level of expansion of space. So the model does not give specific rules like: There are at most 2 or 3 co-planar planes in an atom. Why not?
Because nature does not do that either. Nature simply follows the process of energy optimization which depends on the surrounding especially in the presence of other particles and atoms nearby. So a single isolated C-Atom might look completely different, than a C-Atom within a compound molecule with bonds. This is partially reflected in the following visualization. So a C-Atom which initially might come as a structure of 2 double-planes in 2 different axial orientations might change into a three planar system where the two electrons in the outer shell are not necessarily co-axially entangled anymore, but change into a energetically more optimal structure bonding with electrons of other atoms.
Further simulations might show, whether this model can show local optima which will build up the universe of atoms and molecules with these simple rules.
For further reading on the different attractive and repulsive forces you might be interested in this part: Rotonal Forces